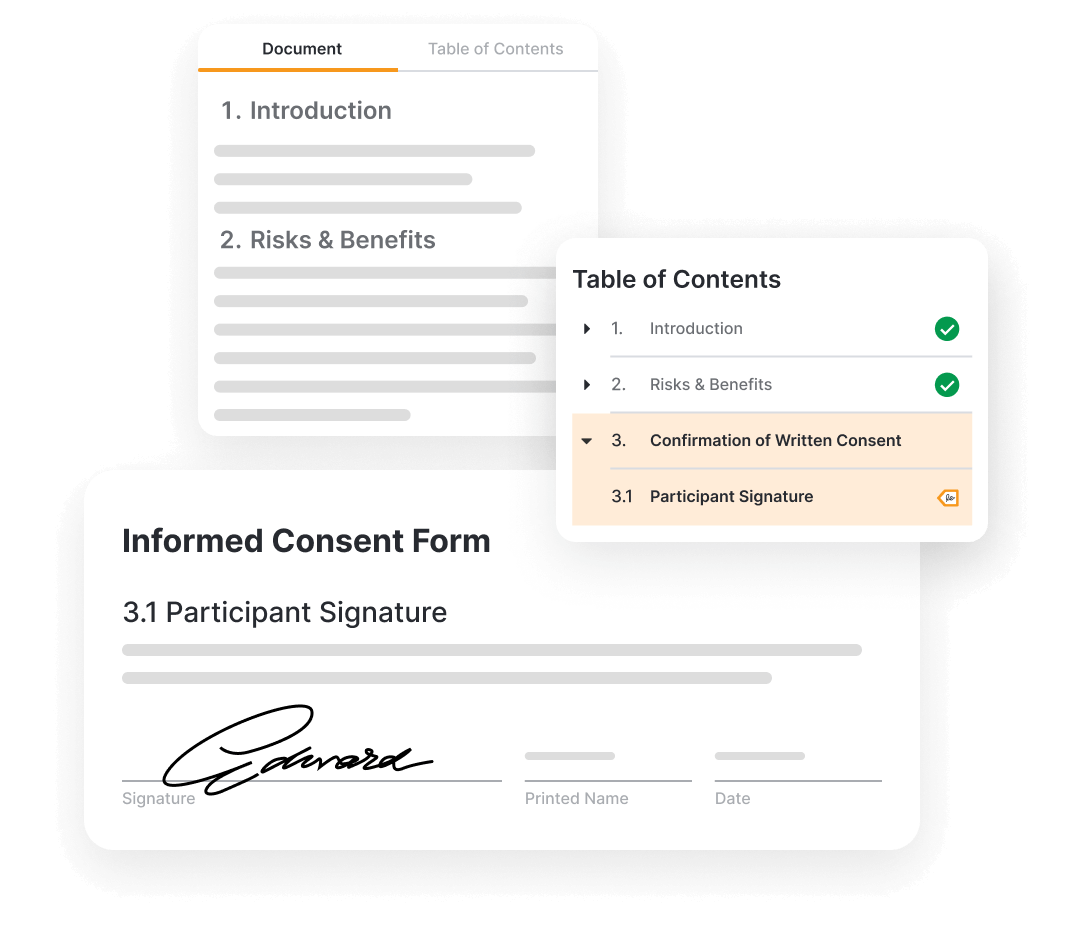
Veeva eConsent provides a digital way to consent clinical trial participants in person or remotely.
Sponsors manage the consent forms in Vault. They can be authored in Word and digitized to include interactive elements like videos, questions, and signatures. Blinded consent data can also be viewed through the sponsors/CRO vault.
Sites are able to review and update consent documents, view participant consent status, and countersign the consents.
Participants, or other signatories consent via MyVeeva for Patients (native application or web). This is also used to access study documents and complete multiple study activities, including eCOA and visit management.