
Integra unifies their clinical platform to establish repeatable processes and provide data visibility
View Customer Story

BD shares how they reduce time wasted for their employees and accelerated the growth of their clinical trials teams
View Customer Story

Illumina exceeds study timelines by leveraging Veeva Clinical Platform to aggregate and clean data from multiple sources
View Customer Story
Complete and concurrent clinical data at all times
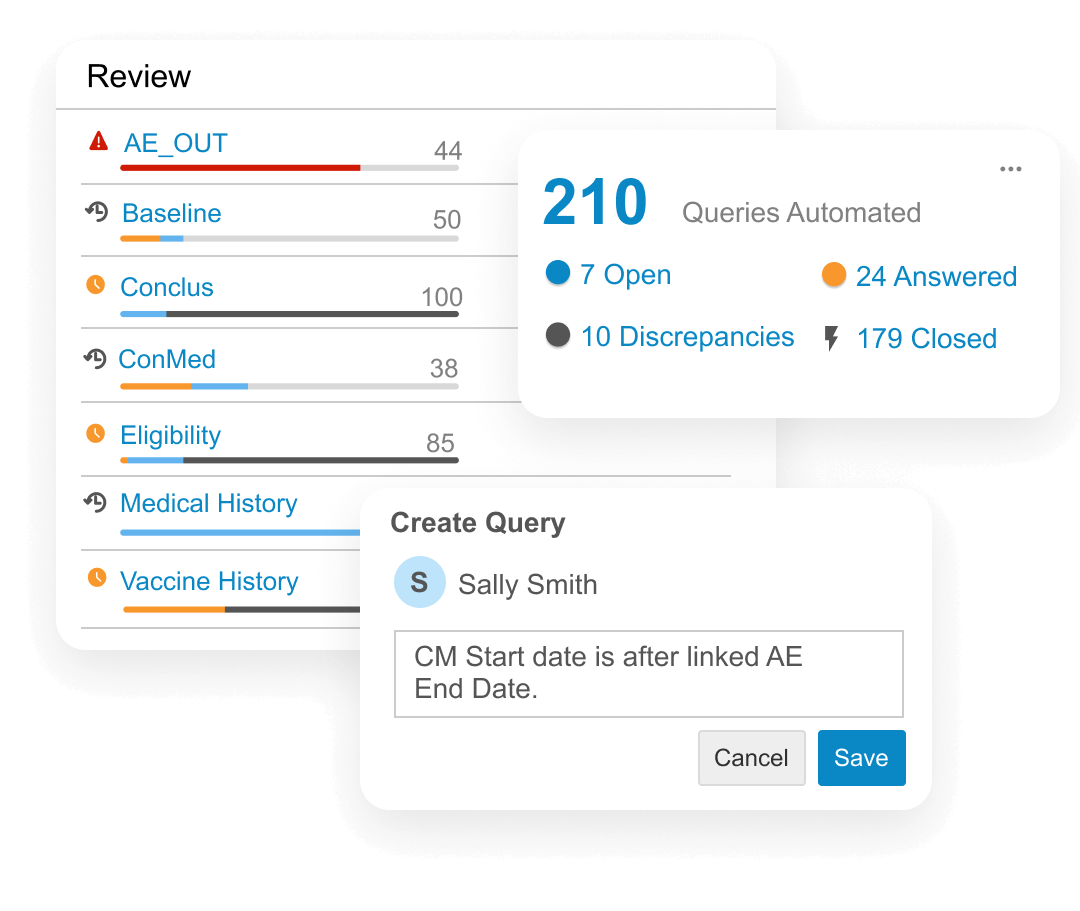
Clinical data managers are collecting data from an increasing number of sources beyond EDC (labs, eCOA, etc). Veeva Clinical Database (CDB) aggregates, cleans, and transforms clinical data from multiple sources, including third-party EDCs.
Data managers access the latest data, assess its status, and track review progress. They log data issues on any source with manual or automated checks and communicate with data providers without switching between EDC, trackers, and emails.
Programmers use Veeva Clinical Query Language (CQL), designed for clinical data, to transform data for reviewers in Veeva CDB or to export data downstream.
30%
cleaning effort saved through automation
50%
easier to generate listings
78%
check queries automatically closed
Veeva CDB Impact
Harmonize clinical data in one place
EDC and third-party data consolidated and aligned to a study backbone data model.Clean without spreadsheet trackers
A comprehensive data workbench allows data managers and providers to action queries in one system.Reduce manual effort
Change detection and automation removes redundant effort and reduces 30-50% of manual tasks.Speed time to database lock
Review and clean all data across sources in one system and create data exports at any time.
See Veeva CDB in action
Customer Success
Medtechs manage complex trials with Veeva Clinical Data
































Watch video
Illumina manages multiple data sources with Veeva CDMS

Watch video
Integra unifies their clinical platform to establish repeatable processes and data visibility

Read article
Alcon shares their experience of working with Veeva EDC and why they prefer this over their prior system

Watch video
BD shares how they reduce time wasted for their employees and accelerated the growth of their clinical trials teams

View infographic
The Clinical Benchmark studies how organizations manage clinical processes, study site collaboration, and trial data

View product brief
Veeva CDB aggregates, cleans, and transforms clinical data from multiple sources, including third-party EDCs





