Announced 2022
Status EARLY
Customers 11-50
Manages GCP and study-specific training
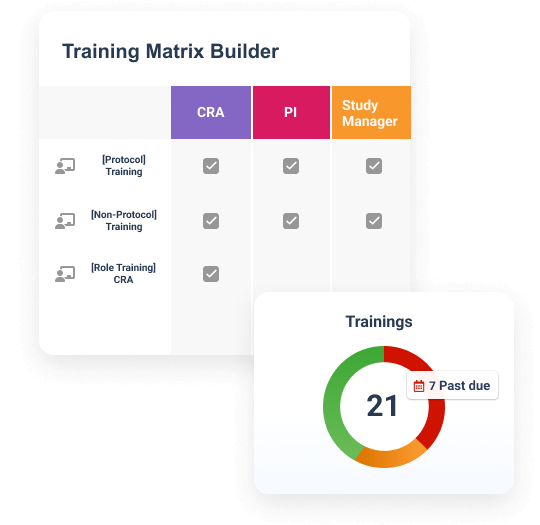
Veeva Study Training manages GCP and study-specific training for research sites, CROs, and sponsor personnel.
Tailored to curricula and training requirements
It provides document, video, and training modules, in addition to quizzes and classroom capabilities based on curricula and training requirements. Teams can create a protocol-specific curriculum, which automatically assigns training based on a user’s role and location. Completed training is documented automatically in an inspection-ready format for study teams and CRAs to leverage.
Connected with Veeva eTMF for inspection readiness
Veeva Study Training connects to Veeva eTMF to eliminate the need to manually capture study and site information.