Veeva Electronic Data Capture (EDC) provides an end-to-end environment to collect, review, and process trial data about patients.
During study start, Veeva EDC is used to design patient forms (including edit checks) without the need for custom programming.
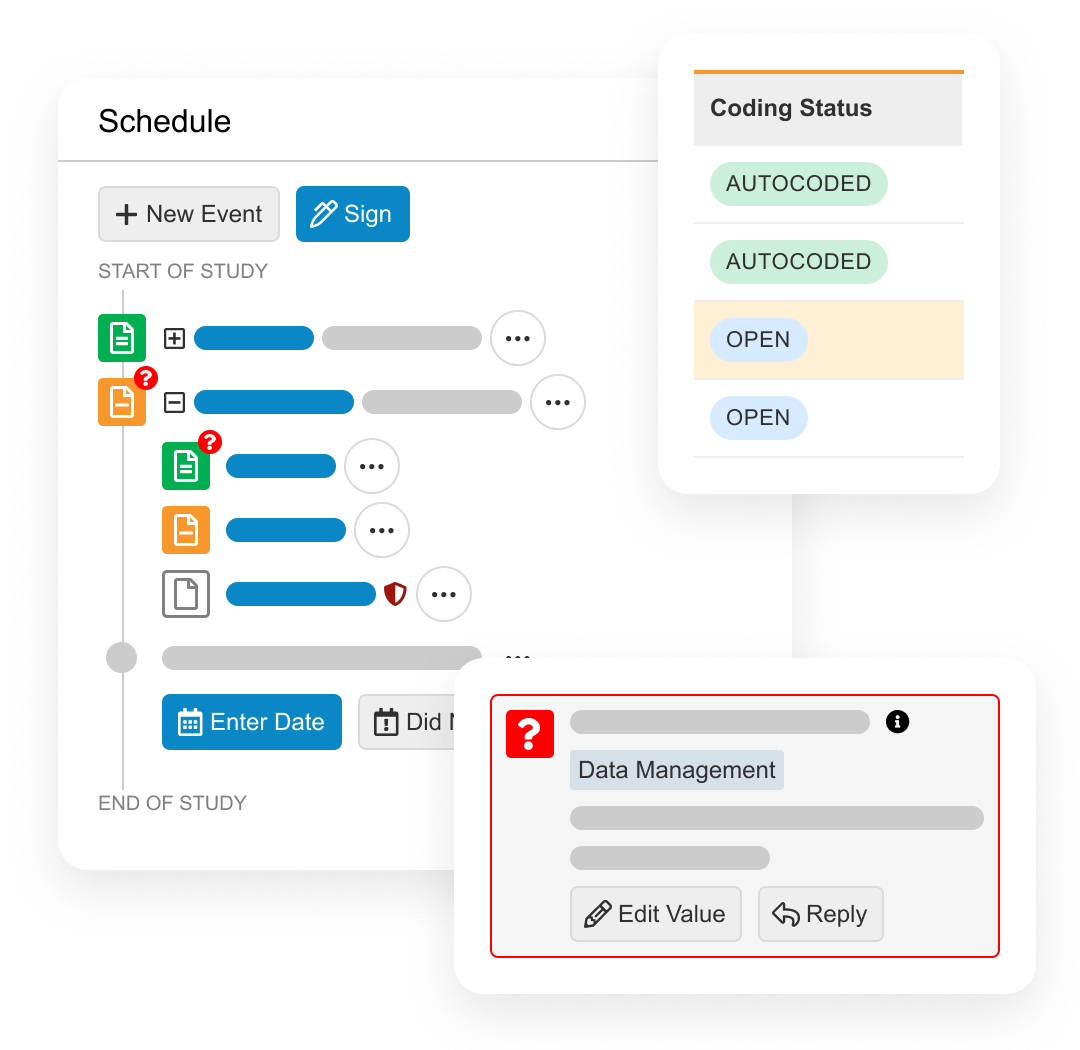
During study execution, Veeva EDC collects all patient form data, local labs, DICOM images, and medical coding. It also has quality controls including querying, targeted source data verification (SDV), and protocol deviations. When protocol amendments happen, the Veeva EDC database needs no downtime.
At the end of the study, Veeva EDC provides data lock and post-processing features, including end-of-study media creation and archiving.