Announced 2016
Status VERY MATURE
Customers 100+
Modernize quality management
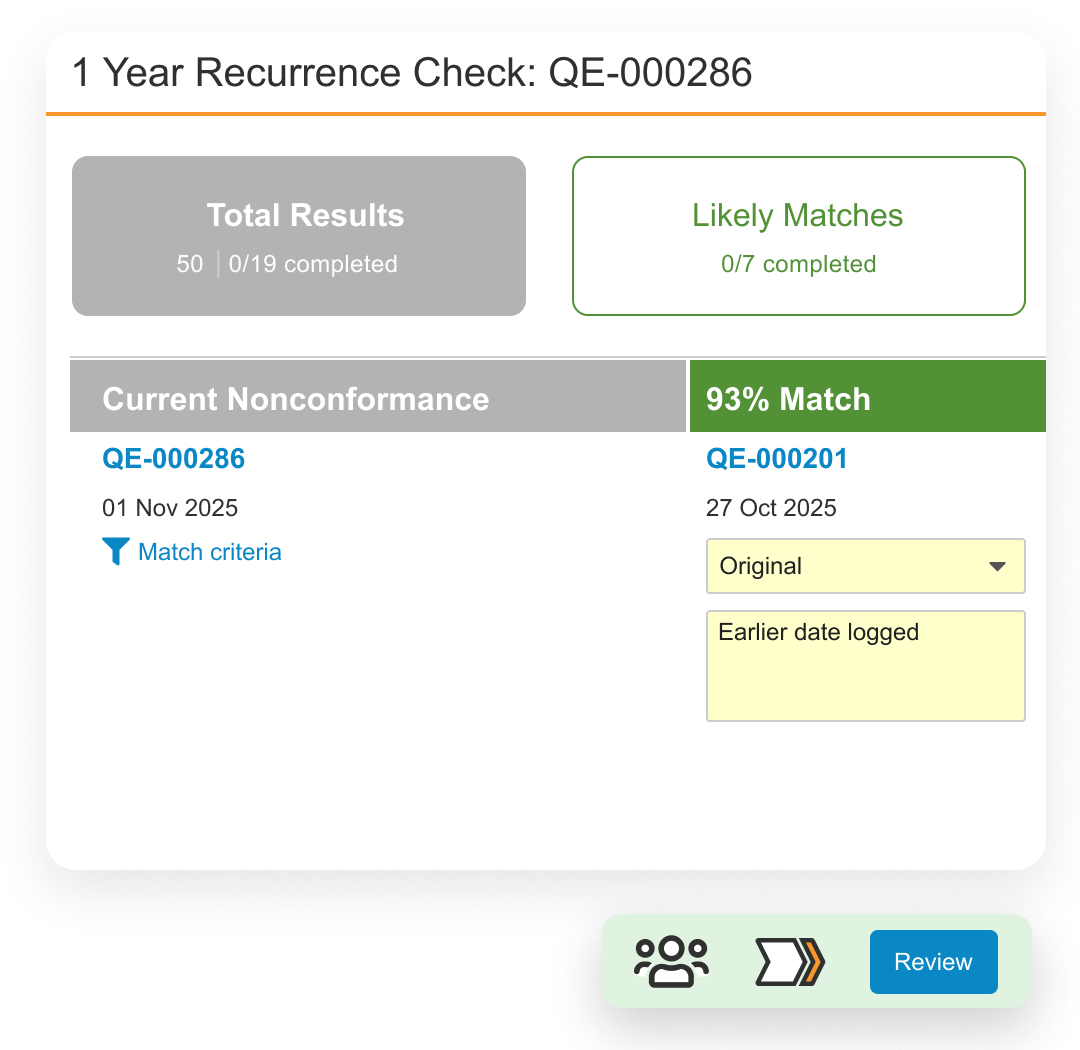
Veeva QMS software provides a unified global quality management system – for all parties – enabling end-to-end process control and visibility for medtech quality teams.
Easily support proactive management initiatives and manage non-conformances, CAPAs, audits, complaints, supplier management, field actions, and change control processes, or configure your own.
Take a holistic approach to view, understand, and manage quality risks and issues to improve decision making.